
C57BL/6N-Crbntm2(CRBN)Bcgen/Bcgen • 113236
在此页面上
Key Advantages
Validation
The coding sequence (CDS) of the human CRBN gene encoding the full-length protein was inserted into mouse Crbn at exons 2–3. The CRBN humanized mice express the human CRBN protein, while mouse Crbn is no longer expressed.
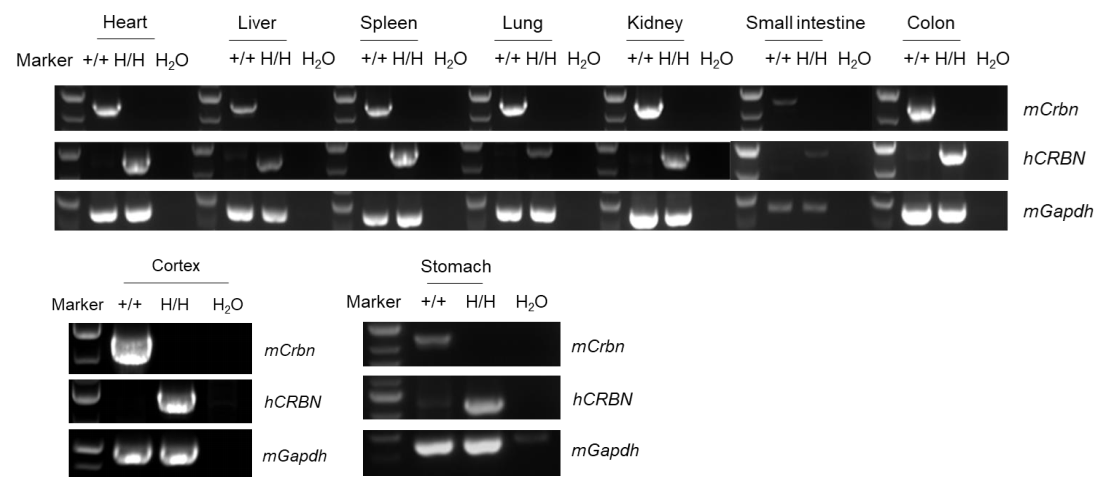
Strain-specific analysis of CRBN mRNA expression in wild-type C57BL/6 mice and homozygous CRBN humanized mice by RT-PCR. Heart, liver, spleen, lung, kidney, stomach, small intestine, colon, and cortex RNA was isolated from wild-type C57BL/6 mice (+/+) and homozygous CRBN humanized mice (H/H). cDNA libraries were synthesized by reverse transcription, followed by PCR with mouse- or human-specific CRBN primers. Mouse Crbn mRNA was detectable in wild-type C57BL/6 mice. Human CRBN mRNA was detectable only in homozygous CRBN humanized mice but not in wild-type mice.

Strain-specific CRBN expression analysis in homozygous CRBN humanized mice by Western blot. Cortex, liver, spleen, lung, kidney, stomach, small intestine, colon, and heart were collected from wild-type C57BL/6 mice (+/+) and homozygous CRBN humanized mice (H/H) and analyzed by Western blot with anti-CRBN antibody (CST, #71810). CRBN was detectable in both wild-type and homozygous CRBN humanized mice. The anti-CRBN antibody was cross-reactive between human and mouse.
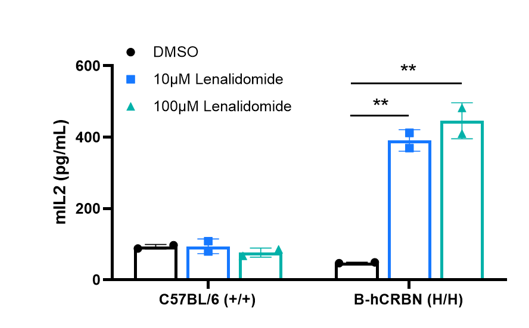
Naive CD4⁺ T cells derived from CRBN humanized mice exhibited increased IL-2 secretion after treatment with lenalidomide. Naive CD4⁺ T cells were collected from wild-type C57BL/6 mice (+/+) and homozygous CRBN humanized mice (H/H), then stimulated with DMSO or lenalidomide (MCE, HY-A0003) in vitro for 24 hours. Supernatants were collected, and IL-2 production was measured by ELISA (Biolegend, 431004). The results show that lenalidomide significantly upregulates IL-2 production in CRBN humanized mice, but not in wild-type mice.
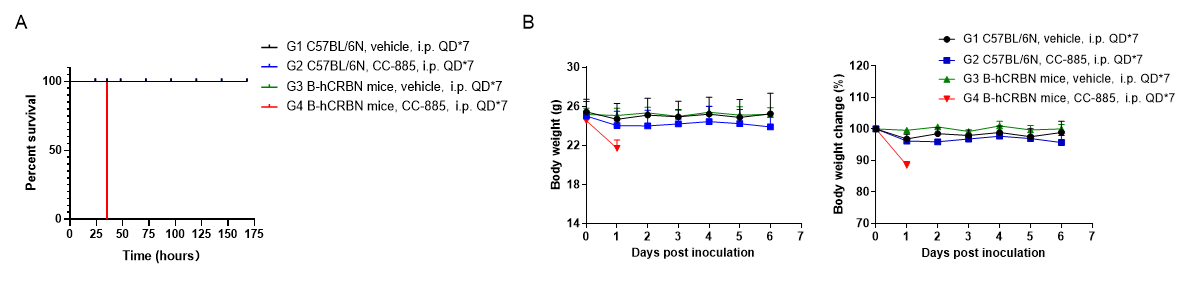
In vivo toxicity of CC-885 in C57BL/6N mice and homozygous CRBN humanized mice. Wild-type C57BL/6 mice and homozygous CRBN humanized mice were each divided into two groups (n = 3), receiving either 5 mg/kg CC-885 (HY-101488) or vehicle via intraperitoneal injection. (A) Percent survival. (B) Body weight and body weight change during treatment. Results demonstrated that all CC-885–treated CRBN humanized mice succumbed at approximately 35 hours post-injection, whereas CC-885–treated wild-type mice and all control groups survived. These results show that CC-885 exhibits marked toxicity exclusively in CRBN humanized mice, with no detectable toxicity in wild-type mice.
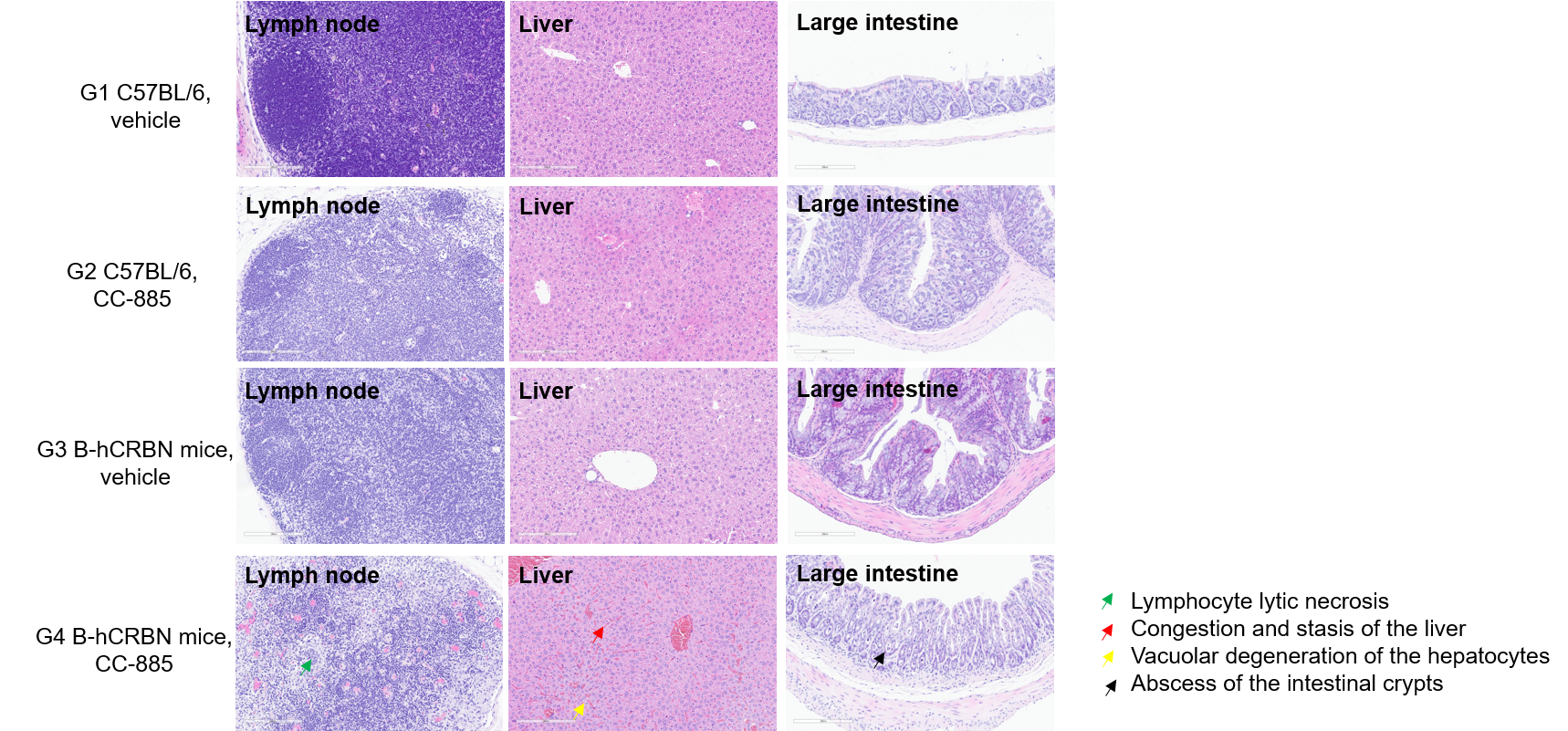
Histopathological analysis was performed on organs from C57BL/6N mice and homozygous CRBN humanized mice. Mice were treated as previously described. Lymph node, liver, and colon tissues were collected from the G4 group 35 hours post-injection; tissues from G1, G2, and G3 groups were sampled at endpoint. As shown in the G4 group, lymph nodes showed lymphocytic lytic necrosis; liver displayed hepatic congestion with stasis and hepatocellular vacuolar degeneration; colon showed intestinal crypt abscesses. No pathological changes were observed in other groups. Scale bar: 100 μm.
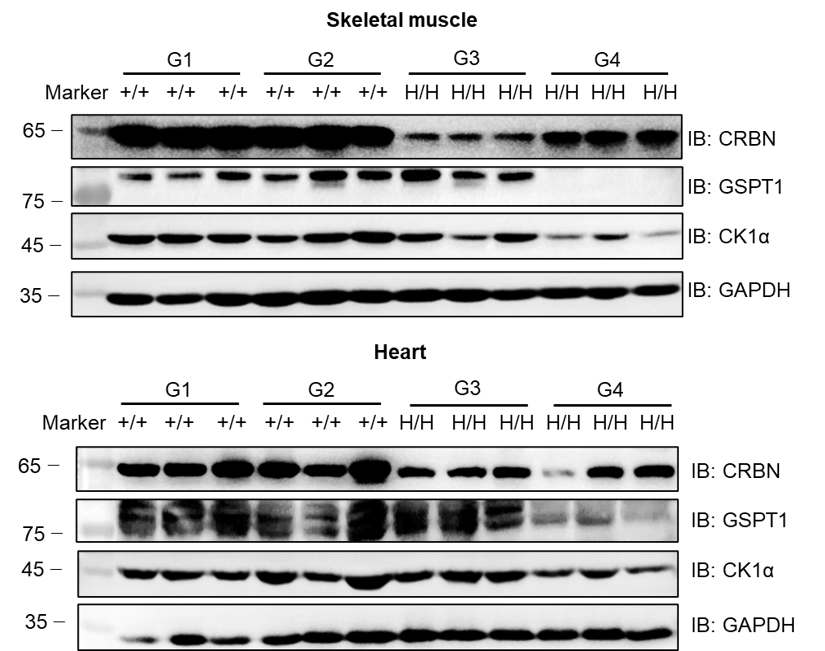
Western blot analysis of GSPT1 protein levels following CC-885 treatment. Skeletal muscle and heart tissue lysates were collected from all groups and analyzed by western blot with anti-CRBN antibody (CST, #71810), anti-GSPT1 antibody (Abcam, ab234433), and anti-CK1α antibody (Abcam, ab302638). 40 μg of total protein was loaded. GSPT1 was detectable in G1, G2, and G3 but not G4. These results demonstrate that CC-885-induced degradation of GSPT1 protein in CRBN humanized mice (G4 group), but not in wild-type mice.
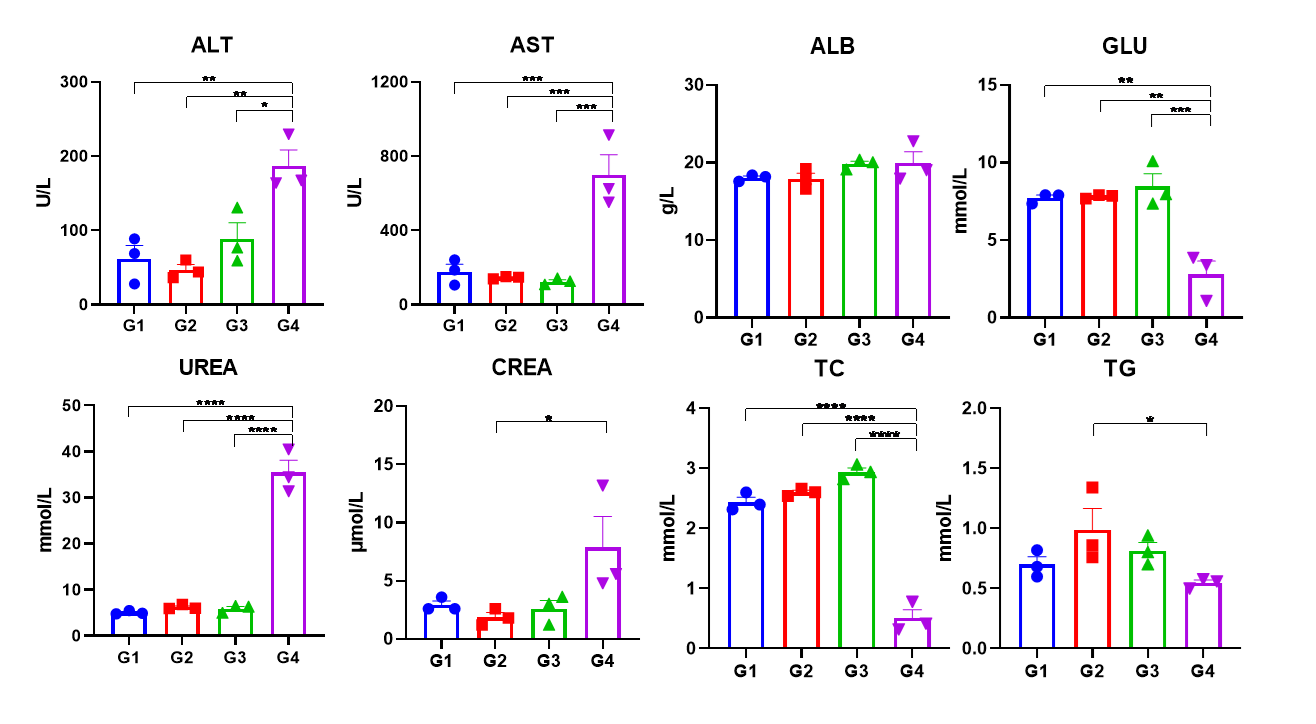
In vivo CC-885 toxicity assessed by blood biochemistry in C57BL/6N and homozygous CRBN-humanized mice. Wild-type C57BL/6 mice and homozygous CRBN humanized mice were each divided into two groups (n=3), receiving either 5 mg/kg CC-885 (HY-101488) or vehicle via intraperitoneal injection. All groups of mice were euthanized for blood collection 6 hours after the second injection, and blood biochemical data (BBD) was measured. Notes:*p<0.05,**p<0.01,***p<0.001,****p<0.0001.
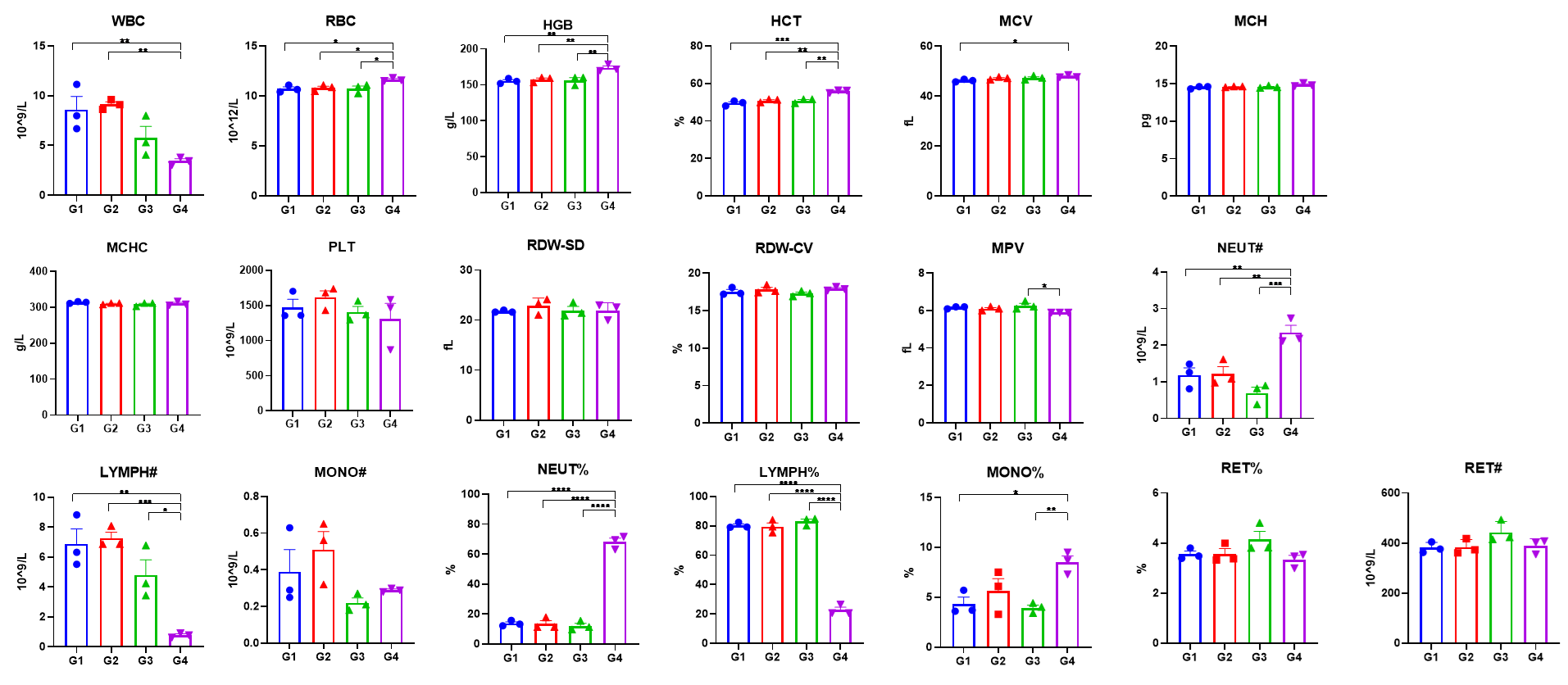
In vivo CC-885 toxicity assessed by complete blood count (CBC) in C57BL/6N and homozygous CRBN-humanized mice. All groups were euthanized 6 hours after the second injection, and CBC indices were measured. Notes: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
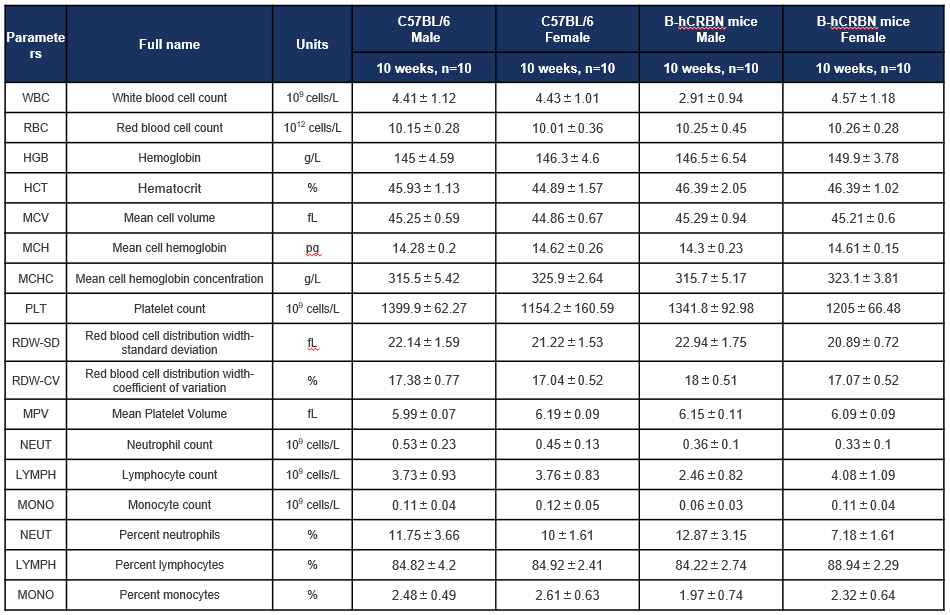
Complete blood count (CBC) of C57BL/6 and CRBN humanized mice. Values are expressed as mean ± SD.
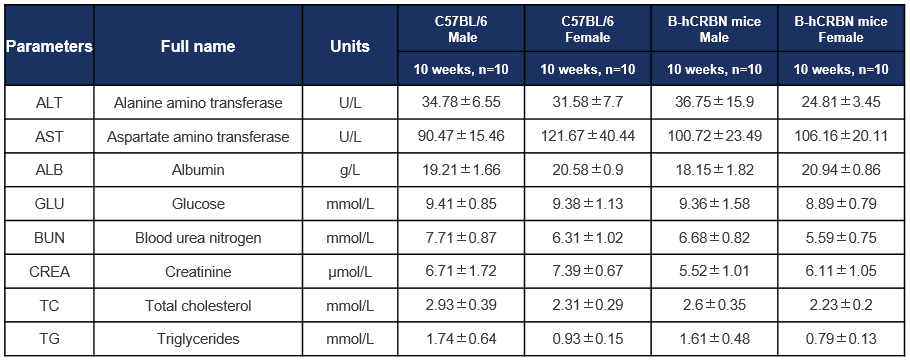
Biochemical test of C57BL/6 and CRBN humanized mice. Values are expressed as mean ± SD.
The organs of female and male C57BL/6 mice (10-week-old, n=10).
The organs of female and male CRBN humanized mice (10-week-old, n = 10).
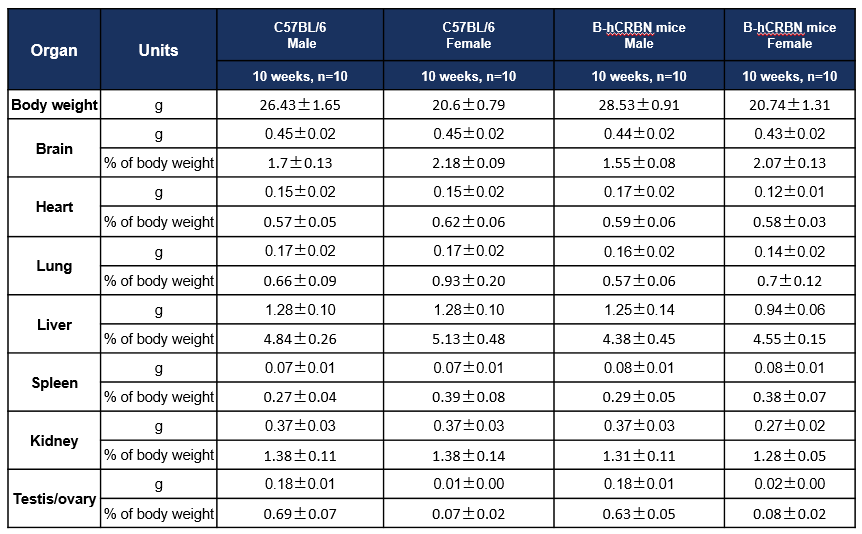
Average weight of major organs in C57BL/6 and CRBN humanized mice. Values are expressed as mean ± SD.
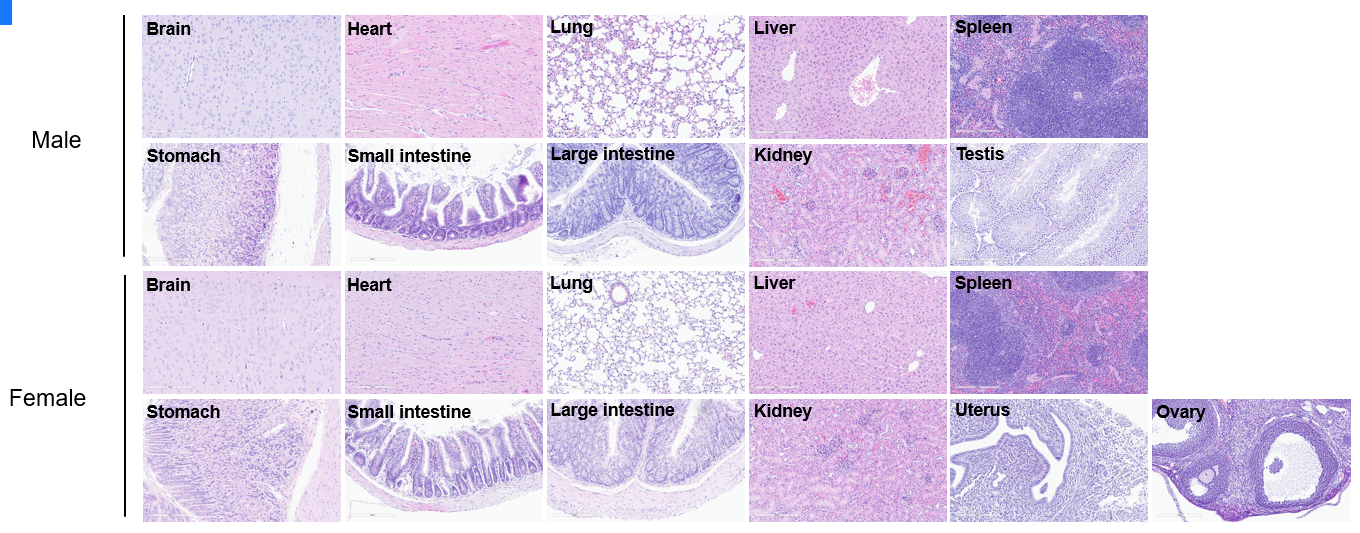
Histopathological analysis of organs in C57BL/6 mice. The main organs of C57BL/6 were isolated at 10 weeks of age and analyzed with H&E staining (male, n=10; female, n=10). Results showed that no obvious abnormalities were found in all of the organs (brain, heart, lung, liver, spleen, stomach, small intestine, colon, kidney, ovary, uterus and testis). Scale bar: 100 μm.
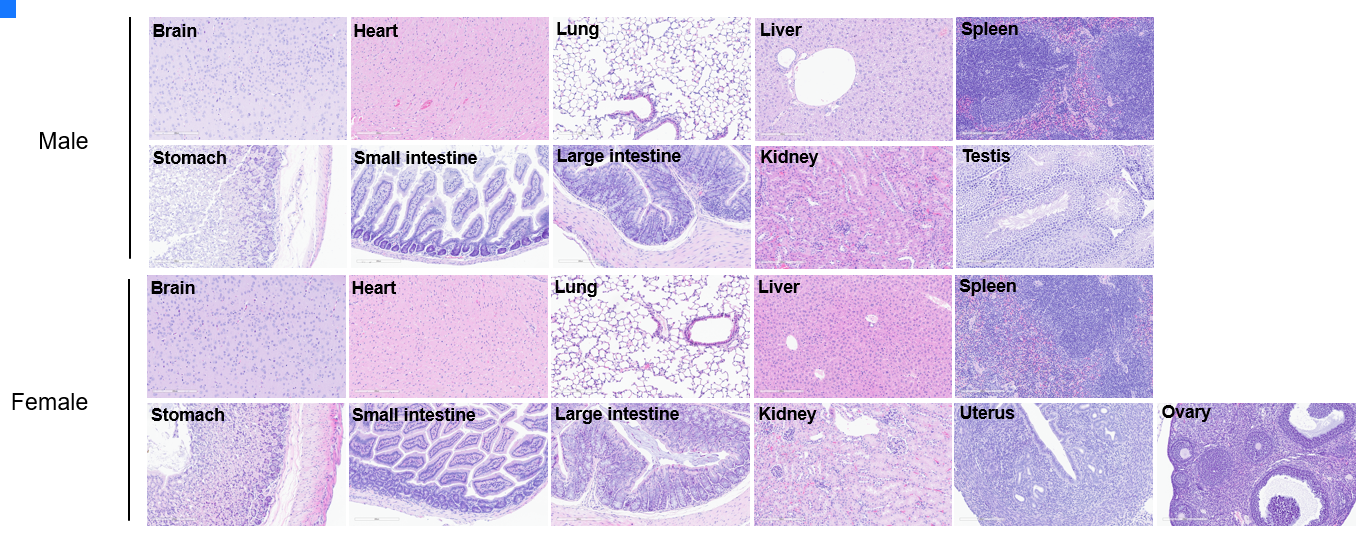
Histopathological analysis of organs in CRBN humanized mice. Major organs were isolated at 10 weeks of age and analyzed with H&E staining (male, n = 10; female, n = 10). No obvious abnormalities were found in all organs examined (brain, heart, lung, liver, spleen, stomach, small intestine, colon, kidney, ovary, uterus, and testis). Scale bar: 100 μm.
Q1: What are CRBN humanized mice used for?
They are designed for evaluating human CRBN-binding drugs, including small molecules, molecular glues, and PROTAC-based degraders, under physiologically relevant human CRBN expression.
Q2: Do CRBN humanized mice exhibit normal baseline physiology?
Yes. CBC, biochemistry, organ weight, and histopathology show no abnormalities, supporting their suitability for long-term studies.
Q3: Why do CRBN humanized mice respond differently to CC-885?
CC-885 selectively binds human CRBN, resulting in degradation of substrates such as GSPT1. This on-target effect occurs only in CRBN humanized mice, not WT animals.
Q4: Can these mice be used for immunomodulatory drug testing?
Yes. Lenalidomide-induced IL-2 upregulation is observed only in CRBN humanized mice, reflecting the human-specific CRBN dependence of its immunomodulatory activity.
Q5: Are CRBN humanized mice suitable for oncology studies?
Absolutely. Tumor cell line inoculation combined with CRBN-dependent therapeutics allows in vivo evaluation of efficacy, safety, and mechanism of action.