| Strain Name |
SD-Rag2tm1Bcgen Il2rgtm1Bcgen/Bcgen
|
Common Name |
B-SDG rats |
| Background | SD | Catalog number | 210512 |
|
Aliases |
Rag2: RAG-2; |
||
|
NCBI Gene ID |
295953,140924 | ||
Description
- The recombinant-activating gene 2 (Rag2) plays an important role in the rearrangement and recombination of the genes of immunoglobulin and T cell receptor molecules during the initiation of V(D)J recombination. Loss of Rag2 protein leads to no mature T and B cells, the critical components of the adaptive immune system.
- Il2rg is known as the interleukin receptor common gamma chain, which is an important signaling component of many interleukin receptors, including those of interleukin -2, interleukin -4, interleukin -7 and interleukin -21. Mutations in this gene cause X-linked severe combined immunodeficiency.
- This rat can be used to support research in many areas including immune system defections, virology research, inflammation research, cancer research, xenograft/transplant host etc.
Targeting strategy
B-SDG rats exhibit the smallest thymus size





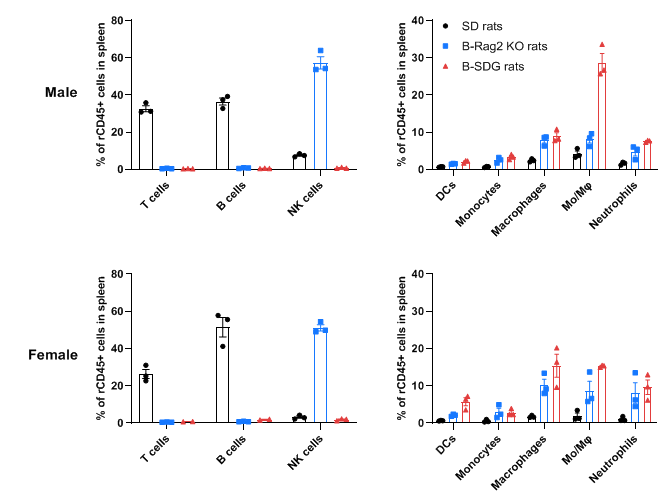
Complete loss of T cells, B cells and NK cells in spleen of homozygous male and female B-SDG rats. Spleens were collected from male and female wild-type SD rats, B-Rag2 KO rats and B-SDG rats (n=3, 7-week-old). Leukocyte subpopulations were analyzed by flow cytometry analysis. Results showed that T cells and B cells were not detectable in spleen of B-Rag2 KO rats and B-SDG rats. NK cells were also not detectable in B-SDG rats. But the percentage of NK cells in B-Rag2 KO rats were relatively higher than that in wild-type SD rats. The percentages of DCs, CD11b+CD43+ monocytes, CD11b+CD68+ macrophages, CD11b+CD43+CD68+ monocytes/macrophages (Mo/Mφ) and neutrophils in B-Rag2 KO rats and B-SDG rats were relatively higher than that in wild-type SD rats. Values are expressed as mean ± SEM.


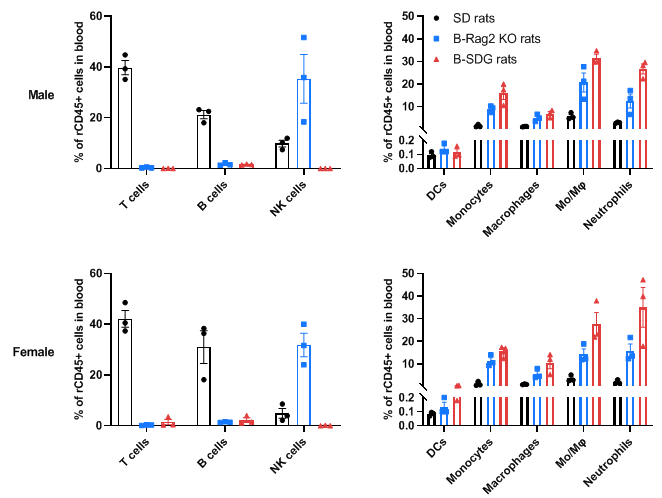
Complete loss of T cells, B cells and NK cells in blood of homozygous male and female B-SDG rats. Blood cells were collected from male and female wild-type SD rats, B-Rag2 KO rats and B-SDG rats (n=3, 7-week-old). Leukocyte subpopulations were analyzed by flow cytometry analysis. A. Representative FACS plots. B. Statistical analysis of FACS. Results showed that T cells and B cells were not detectable in blood of B-Rag2 KO rats and B-SDG rats. NK cells were also not detectable in B-SDG rats. But the percentage of NK cells in B-Rag2 KO rats were relatively higher than that in wild-type SD rats. The percentages of DCs, CD11b+CD43+ monocytes, CD11b+CD68+ macrophages, CD11b+CD43+CD68+ monocytes/macrophages (Mo/Mφ) and neutrophils in B-Rag2 KO rats and B-SDG rats were relatively higher than that in wild-type SD rats. Values are expressed as mean ± SEM.
Growth curve
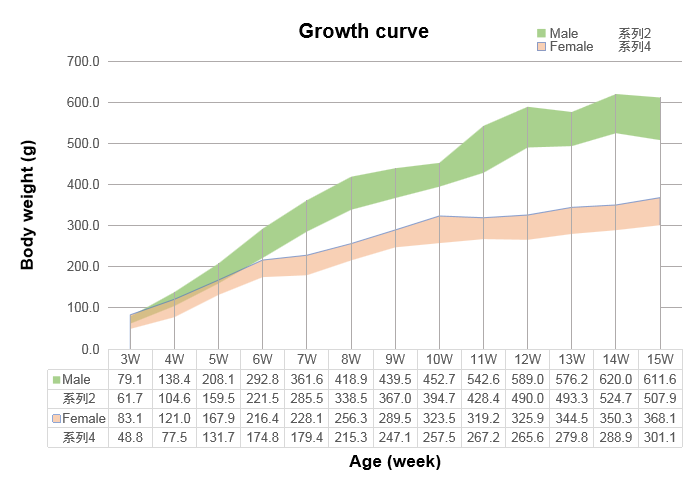
Growth curve of B-SDG rats.Three weeks old of newborn pups were obtained at weaning. Body weight of the rats with ages from 3 weeks to 15 weeks was measured (male: n=14~21; female: n=21~30). The lowest and highest values of the body weight in the table were calculated from the mean ± SD. The growth curve conforms to the normal distribution and the probability of random error falling within ± SD is 68%.

Subcutaneous homograft tumor growth of NCI-H460 cells in B-SDG rats. Human large cell lung cancer cell line NCI-H460 were mixed with Matrigel (1:1, Corning, 354230) and inoculated subcutaneously into B-SDG rats (female, 9-week-old, n=6). (A)Tumor volume. (B) Body weight change. As shown in panel A, NCI-H460 cells were able to establish tumors in B-SDG rats, and this strain of rat can be used for efficacy studies.

Subcutaneous homograft tumor growth of MIA PaCa-2 cells in B-SDG rats. Human pancreas cancer cell line MIA PaCa-2 (2x107) were mixed with Matrigel (1:1, Corning, 354230) and inoculated subcutaneously into B-SDG rats (n=6). (A)Tumor volume. (B) Body weight. As shown in panel A, MIA PaCa-2 cells were able to establish tumors in B-SDG rats and B-Rag2 KO rats, and the two strains of rats can be used for efficacy studies.
In vivo efficacy of cisplatin evaluated with B-SDG rats
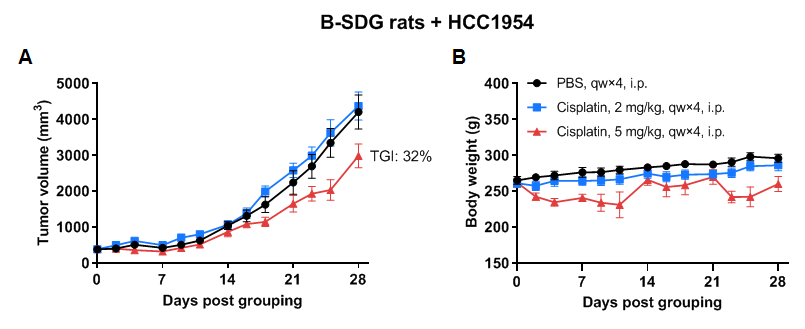
In vivo efficacy of cisplatin evaluated with B-SDG rats
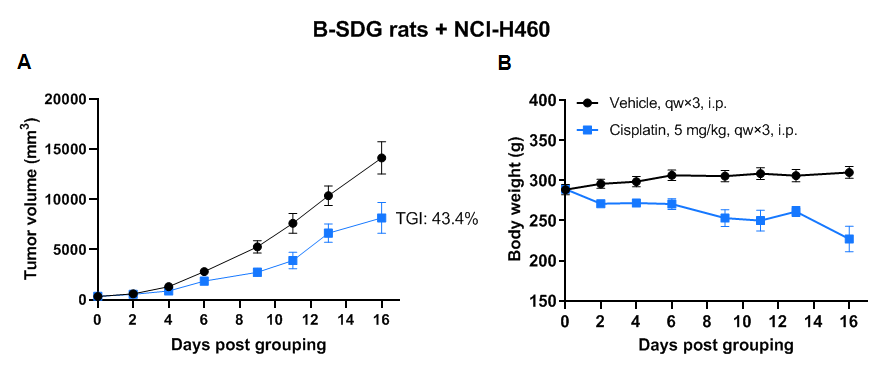
Antitumor activity of cisplatin in B-SDG rats. (A) Cisplatin inhibited NCI-H460 tumor growth in B-SDG rats. Human large cell lung cancer cell line NCI-H460 (2E6) mixed with Matrigel (1:1, Corning, 354230) were subcutaneously implanted into B-SDG rats (female, 9-week-old, n=6). The rats were grouped when tumor volume reached approximately 300-400 mm3, at which time they were treated with cisplatin with the dose indicated in panel. (B) Body weight changes during treatment. As shown in panel A, cisplatin was efficacious in controlling tumor growth in B-SDG rats, demonstrating that B-SDG rats provide a powerful preclinical model for in vivo evaluation of chemotherapies. Values are expressed as mean ± SEM.
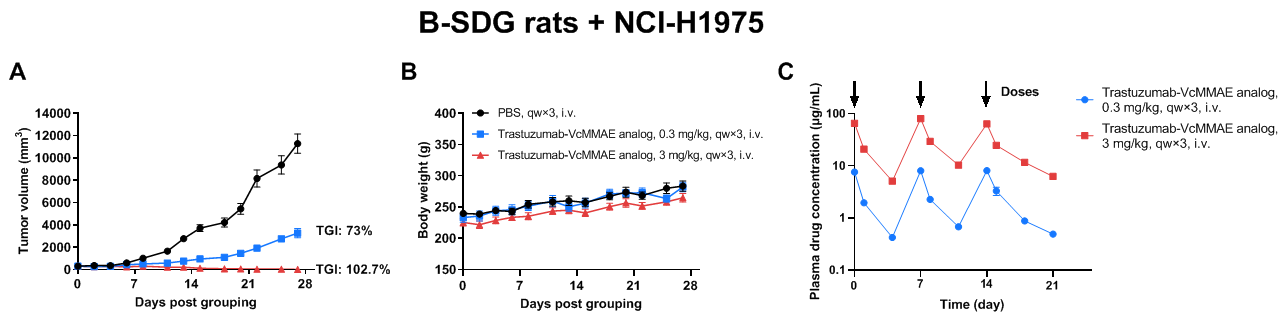
Antitumor activity of Her2-targeting ADC drug in B-SDG rats. Human non-small cell lung cancer cell line NCI-H1975 (5×106) mixed with Matrigel (1:1, Corning, 354230) were subcutaneously implanted into B-SDG rats (female, 8-week-old, n=6). The rats were grouped when tumor volume reached approximately 300-400 mm3, at which time they were treated with trastuzumab-VcMMAE analog (in-house). Blood was collected at 15 min, 24 hr and 96 hr after the first two doses from every mouse. After the third dose, the time points of blood collection were 15 min, 24 hr, 96 hr and 168 hr. (A) Trastuzumab-VcMMAE analog (in-house) inhibited the growth of NCI-H1975 in B-SDG rats. (B) Body weight changes during treatment. (C) Time course of drug concentration in blood. Results showed that Her2-targeting ADC drug was efficacious in controlling tumor growth in B-SDG rats in a dose-dependent manner. The drug concentration was maintained at a steady high level in the high dosing group. There is a significant correlation between the efficacy and drug concentration. Therefore, B-SDG rats provide a powerful preclinical model for in vivo efficacy evaluation and pharmacokinetics study of ADC drugs. Values are expressed as mean ± SEM.












 京公网安备:
京公网安备: